Scrambler Therapy Introduction

Scrambler Therapy is a proven, unique, non-invasive method for rapid treatment of chronic intractable neuropathic and cancer-related pain.
Who is it for?
Scrambler Therapy is a new electroanalgesia methodology studied explicitly for neuropathic pain, oncologic pain, and, in general, for pain non-responsive to other types of drugs and forms of electrical stimulation therapies (TENS, spinal cord stimulator)
How Scrambler Therapy® Technology Works?
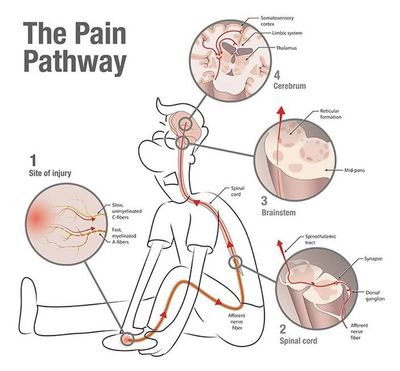
Five independent channels are available to transmit the artificial “no pain” messages via surface electrodes (non-invasive) placed on the skin outside the region of the patient’s pain. Protocols are different based on the types of pain treated. It stimulates C fibers (pain carrying fibers) in a physiological form vs. TENS unit excessively stimulates A-Beta fibers to suppress signal transmission by C-fibers.
History of Scrambler Therapy
It was invented by Prof. Giuseppe Marineo around 2003 at the University of Rome, Italy. It received FDA clearance in 2009.
What is the efficacy of Scrambler Therapy?
It is generally operator and patient (type of condition) dependent. In clinical trials of CINP (Chemotherapy Induced Peripheral Neuropathy), efficacy has been around 60%. However, they can be higher than 80%.
What are the effects of combinations with other analgesic therapies?
Scrambler Therapy is a stand-alone medical electroanalgesia device and does not require combinations with other analgesic therapies. Using anticonvulsants for analgesic purposes generally calls for a higher number of treatments. It is also possible to continue the anticonvulsant analgesic therapy; however, in this case, results are not as good, and relapse is quicker. From the study phase data, the combination with Ketamine is incompatible since it seems to block the analgesic efficacy of the treatment.
Indications for Use
Painful Peripheral Neuropathy
Complex Regional Pain Syndrome (CRPS / RSD)
Chemotherapy-Induced Peripheral Neuropathy (CIPN)
Intractable Cancer Pain
Failed Back Surgery Syndrome
Sciatic and Lumbar Pain
Post-herpetic neuralgia (PHN)
Brachial Plexus Pain
Chronic Neuropathic Pain, Allodynia, Hyperalgesia
Scrambler Therapy® Technology Treatment Protocol
Additional Information
The patient visits the practice for ten treatment sessions on average—one session per day over a period of two weeks (weekdays). Treatment sessions may last from 30 to 45 minutes. Booster cycles are given when needed for symptom recurrence.
During the first session, after the correct electrode positioning and fine-tuned stimulation levels, the patient may notice significant improvements quickly. In the subsequent sessions, the patient may realize that pain relief is prolonged (reduced intensity and duration). So far, Scrambler Therapy does not have any known side effects except mild irritation at the site of electrode placement
Scrambler Therapy Approval
Scrambler Therapy® Technology MC-5A is a pain therapy medical device that is U.S. FDA 510(k) cleared (# K142666) and European CE mark-certified (#CE 0470).
Scrambler Therapy Used At
The Scrambler Therapy device has been actively used at MD Anderson Cancer Center, Mayo Clinic, John Hopkins, MUSC (Medical University of South Carolina), and Walter Reed Army Hospital.
Scrambler Therapy at Major Institutions
Reference Articles Online
Reference Website
Scrambler Therapy Official Information Website.

Bibliography - Reference Research Articles
Untreatable pain resulting from abdominal cancer: new hope from biophysics?
JOP. 2003 Jan;4(1):1-10.
Scrambler therapy (PMID:16012423) Minerva Anestesiologica [01 Jul 2005, 71(7-8):479-482]
Pilot trial of a patient-specific cutaneous electrostimulationdevice (MC5-A Calmare®) for chemotherapy-induced peripheral neuropathy
J Pain Symptom Manage. 2010 Dec;40(6):883-91. doi: 10.1016/j.jpainsymman.2010.03.022.
Managing chronic pain: results from an open-label study using MC5-A Calmare® device.
Support Care Cancer. 2012 Feb;20(2):405-12. doi: 10.1007/s00520-011-1128-6. Epub 2011 Mar 11
J Pain Symptom Manage. 2012 Jan;43(1):87-95. doi: 10.1016/j.jpainsymman.2011.03.015. Epub2011 Jul 16.
Scrambler Therapy for Patients with Cancer Pain - Case Series
Korean J Pain. 2013 Jan; 26(1): 65–71. Published online 2013 Jan 4. doi: 10.3344/kjp.2013.26.1.65
Clinical Experiences on the Effect of Scrambler Therapy for Patients with Postherpetic Neuralgia
Korean J Pain. 2013 Jan; 26(1): 98–101. Published online 2013 Jan 4. doi: 10.3344/kjp.2013.26.1.98
Decreased low back pain intensity and differential gene expression following Calmare®: results from a double-blinded randomized sham-controlled study.
Res NursHealth. 2015 Feb;38(1):29-38. doi: 10.1002/nur.21632. Epub 2015 Jan 8.
Chronic pain treatment and scrambler therapy: a multicenter retrospective analysis. ActaBiomed. 2015 Sep 14;86(2):149-56.
Pilot evaluation of scrambler therapy for pain induced by bone and visceral metastases and refractory to standard therapies. Support Care Cancer. 2016 Apr;24(4):1649-54. doi: 10.1007/s00520-015-2952-x. Epub2015 Sep 26.
An exploratory study on the effectiveness of "Calmaretherapy" in patients with cancer-related neuropathic pain: A pilot study. EurJ Oncol Nurs. 2016 Apr;21:1-7. doi: 10.1016/j.ejon.2015.12.001. Epub2015 Dec 29.
Predictive factors associated with success and failure for Calmare(Scrambler) therapy: a multicenter analysis. Clin J Pain. 2015 Aug;31(8):750-6. doi: 10.1097/AJP.0000000000000155
Scrambler (Calmare) Therapy for Intractable Chronic Pain Soo A Kim Soonchunhyang Medical Science 21(2):169-172, December 2015 pISSN: 2233-4289 I eISSN: 2233-429
Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2015 Apr;23(4):943-51. doi: 10.1007/s00520-014-2424-8. Epub2014 Sep 24.
An Expanded Trial of Scrambler Therapy in the Treatment of Cancer Pain Syndromes And Chronic Chemotherapy-Induced Peripheral Neuropathy J Pain PalliatCare Pharmacother. 2013 Dec; 27(4): 359–364. Published online 2013 Oct 21. doi: 10.3109/15360288.2013.847519
Scrambler Therapy for the management of chronic pain. Support Care Cancer. 2016 Jun;24(6):2807-14. doi: 10.1007/s00520-016-3177-3. Epub 2016 Apr 4
Scrambler Therapy(®) MC-5A for Complex Regional Pain Syndrome: Case Reports. Pain Pract. 2016 Sep;16(7):E103-9. doi: 10.1111/papr.12474. Epub2016 Jul 2.
Impact of Scrambler Therapy on Pain Management and Quality of Life in Cancer Patients: A Study of Twenty Cases Indian J PalliatCare. 2017 Jan-Mar;23(1):18-23. doi: 10.4103/0973-1075.197948.
Effect of pain scrambler therapy on antineuralgic pain and quality of life after shingles. J Phys Ther Sci. 2017 Jun; 29(6): 1113–1115.
Scrambler therapy for the treatment of neuropathic pain related to leukemia in a pediatric patient: A case report. Medicine (Baltimore). 2017 Nov;96(45):e8629. doi: 10.1097/MD.0000000000008629.
Treatment of Postherpetic Pain With Scrambler Therapy, a Patient-Specific NeurocutaneousElectrical Stimulation Device. Am J Hosp PalliatCare. 2018 May;35(5):812-813. doi: 10.1177/1049909113494002. Epub 2013 Jul 8.
Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: A preliminary study. Pediatr Blood Cancer. 2018 Jul;65(7):e27064. doi: 10.1002/pbc.27064. Epub 2018 Apr 6
Scrambler therapy: what's new after 15 years? The results from 219 patients treated for chronic pain Medicine (Baltimore). 2019 Jan; 98(2): e13895
Case Report: Scrambler Therapy for Treatment-Resistant Central Neuropathic Pain in a Patient with Transverse Myelitis. IntJ MS Care. 2019 Mar-Apr;21(2):76-80. doi: 10.7224/1537-2073.2017-083.
Inside the Scrambler Therapy, a Noninvasive Treatment of Chronic Neuropathic and Cancer Pain: From the Gate Control Theory to the Active Principle of Information. IntegrCancer Ther. 2019 Jan-Dec;18:1534735419845143. doi: 10.1177/1534735419845143.
